Evaluating New Drugs with Wearable Technology
Can wearables and electronic data capture (EDC) technologies improve clinical trials and accelerate new drug approval?
The drug development process is a long, tedious, and expensive journey. A pharmaceutical company (“pharma”) can expect to spend on average 10 years and $2.5 billion to complete a clinical trial.1,2,3
The regulatory process for approval of a new drug
The Food and Drug Administration (FDA) is the governing body that regulates drug and biologic approval. The FDA requires pharma to perform three human clinical phase trials, Phase I-III, before applying for a license to sell that drug. Phase I trials demonstrate drug safety in fewer than 100 healthy volunteers, while phase II studies evaluate drug efficacy in hundreds of patients.4,5 Phase III clinical trials are the most complex, costly, and time-consuming because they include thousands of patients.4,5 Upon successful completion of Phase III clinical trials a company may file a New Drug Application with the FDA.4,5 If the application is approved, that company can sell and market that drug in the USA. Phase IV trials occur after drug commercialization.5
Since only 12% of drug applications are approved by the FDA, clinical trials are risky, costly, and lengthy and can result in billion dollar gains or losses, depending on whether the drug receives approval.1,2,6 These odds are not in pharma’s favor. To fuel continued growth pharma must strategically invest in the development and acquisition of innovative biologics and personalized medicines to bolster their drug pipelines (Figure1). In addition, there is mounting pressure for pharma to collaborate with clinical research organizations (CROs, companies that design and conduct clinical trials) to improve clinical trial efficiencies by decreasing costs and timelines, improving quality and safety, and gathering more data that better informs trial endpoints.2,6

Improving the clinical trial process with EDC
A government sponsored study recently identified several strategies in which pharma and CROs are mitigating clinical trial barriers including the use of electronic records, simplified SOPs, and lower-cost facilities, among others.6 Of these strategies, the wider use of electronic data capture (EDC) through the use of smartphones and wearables, like Fitbits, will dramatically disrupt how clinical trials are designed and executed creating value to all stakeholders.6,7
How does EDC pertain to clinical trials? EDC refers to software that electronically collects, stores, and manages clinical and laboratory data.8,9,19 As an end-to-end management system, EDC systems have been implemented by the large CROs and are estimated to be a $2-5 billion market by 2020.10,11,12 EDC systems have improved clinical trial management by improving recruitment, statistical analysis, site monitoring timelines and costs, site management, data collection, data security, data accessibility, and error rates.6,13,14.19 Theses efficiencies have resulted in a 17.6% decrease in trial timelines and a 9.8% decrease in trial costs.6 These are big wins for pharma and consumers.
Wearable devices in the evaluation of new drugs
While EDCs have proven their worth in improving clinical trial data management, Litmus Health (“Litmus”) is taking it a step further by using their clinical trial science platform to enable trial sponsors to make better go/no-go decisions during Phase I-II clinical trials.15,16,17 The company’s technology collects patient data from wearables, smart devices, and home sensors and then uses algorithms to align and integrate data from multiple patients at multiple time-points with the goal of identifying trends in patient outcomes, quality of life, environment, etc. (Figure 2).11,15,16 By having access to and a better understanding of the trends observed in Phase I-II trails, trial sponsors and CROs are able to design a leaner, and subsequently, more time- and cost-efficient Phase III trial. The Litmus technology is new to the market, being pilot tested in a clinical trial at the University of Chicago.15,18 The real-time sleep and activity data from patients suffering from Inflammatory Bowel Syndrome will be used to personalize treatment and manage disease symptoms.15,18
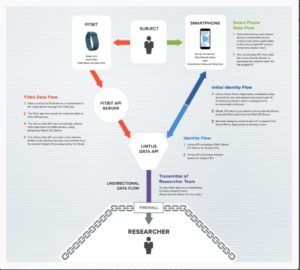
The advent of Litmus’ data collection and analysis technology will transform the way clinical trials are designed and performed with the goal of advancing drugs with the greatest human health impact factor. Conversely, these datasets may empower pharma to pull the plug on clinical trials earlier if a drug is ineffective or unsafe which could save time and money. However, with limited clinical trial exposure, Litmus faces several hurdles to technology awareness, adoption, and implementation that could be delayed due to inherent factors in the clinical trial process including limited IT infrastructure, workflow of healthcare professionals, lack of training, and up-front costs.9 Furthermore, the strength of this technology is in its ability to collect and analyze large amounts of data, but will all this data prove useful or only a small subset? Litmus should consider evaluating multiple data points and sources with CRO partners to determine the types of data that bring value in actual clinical trials. Only time will tell the outcome, but it is likely that Litmus’ souped-up EDC system will disrupt how clinical trials are performed. (795 words)
REFERENCES
- Dimasi, J., Grabowshi, H.G., and Hansen, RW. Innovation in the pharmaceutical industry: New estimates of R&D costs. Journal of Health Economics. 2016. 47: 20-33.
- Pharmaceutical Research and Manufacturers of America. 2015 biopharmaceutical research industry profile. Washington, DC: PhRMA; April 2015. http://www.phrma.org/sites/default/files/pdf/2015_phrma_profile.pdf
- Feyman, Y. Shocking Secrets Of FDA Clinical Trials Revealed. Forbes. Jan 24, 2014. http://www.forbes.com/sites/theapothecary/2014/01/24/shocking-secrets-of-fda-clinical-trials-revealed/#703a333a2279
- http://www.fda.gov/downloads/drugs/resourcesforyou/consumers/ucm284393.pdf (Figure 1)
- National Library of Medicine, National Institutes of Health. https://www.nlm.nih.gov/services/ctphases.html.
- Sertkaya, et al. Eastern Research Group. Examination of Clinical Trial Costs and Barriers for Drug Development. ASPE. US Department of Health and Human Services. https://aspe.hhs.gov/report/examination-clinical-trial-costs-and-barriers-drug-development
- Field, A. Where the action is in wearbles: Healthcare niches. Cisco. August 01, 2016. https://newsroom.cisco.com/feature-content?type=webcontent&articleId=1781211
- Kush. R. Electronic Data Capture-Pros and Cons. BioExecutive International. June 2006. https://www.cdisc.org/system/files/all/reference_material/application/pdf/bei26kushsup.pdf.
- Parekh, S. Electronic Data Capture in Clinical Trials. Applied Clinical Trials. September 1, 2013. Vol 22, Issue 9. http://www.appliedclinicaltrialsonline.com/electronic-data-capture-clinical-trials
- eClinical Solutions Director of Electronic Data Solutions Honored in PharmaVOICE 100.FierceHealthcare.August 2, 2011, http://www.fiercehealthcare.com/it/eclinical-solutions-director-electronic-data-solutions-honored-pharmavoice-100.
- Fassbender, M. Early stage clinical trials get boost from modern EDC platform. Outsourcing Pharma. Nov 2, 2016. http://www.outsourcing-pharma.com/Clinical-Development/Litmus-Health-launches-modern-EDC-platform (Figure 2)
- Stanton, D. Medidata chasing an every-increasing slice of the $2bn EDC market. Outsourcing Pharma. April 27, 2015. http://www.outsourcing-pharma.com/Commercial-Services/Medidata-chasing-greater-share-of-2bn-EDC-market
- Electronic Data Capture/Clinical Trial Management Systems – Smartphone apps for clinical trials. FierceBiotech. http://www.fiercebiotech.com/special-report/electronic-data-capture-clinical-trial-management-systems-smartphone-apps-for
- Lopienski, K. The Beginner’s Guide to an Electronic Data Capture (EDC) System. Forte Research Systems. Feb 2, 2016. http://forteresearch.com/news/beginners-guide-electronic-data-capture-edc-system/
- Litmus Health Launches Clinical Data Science Platform Focused on Health-Related Quality of Life.prweb.October 27, 2016. http://www.prweb.com/releases/2016/10/prweb13799965.htm
- Baum, S. Litmus Health uses wearables to collect patient data for clinical trials. Med City News. October 27, 2016. http://medcitynews.com/2016/10/litmus-health-launches-adding-digital-health-companies-supporting-clinical-trials/
- http://www.litmushealth.com/ (cover image photo)
- Tracking real-time data to personalize treatment for IBD. ScienceLife.May 17, 2016, https://sciencelife.uchospitals.edu/2016/05/17/tracking-real-time-data-to-personalize-treatment-for-ibd/.
- Fundamental Aspects of Electronic Data Capture.OpenClinica. assessed on November 16, 2016. https://www.openclinica.com/electronic_data_capture/



Tarran this is such an interesting article, reminding me of several cases we had on pharmaceuticals! It is amazing how digital transformation can make an impact on not just drug development process but also its approval process. You pointed out many challenges that Litmus faces such as “limited IT infrastructure, workflow of healthcare professionals, lack of training, and up-front costs.” I believe data accuracy can be an issue itself too. Not to mention the controversy around Fitbit’s measurement accuracy (http://www.nytimes.com/2016/05/26/technology/personaltech/fitbit-accuracy.html?_r=0), manual data input and survey completion by test participants can potentially deteriorate the quality of data compared to more controlled, direct measurement. As you mentioned, until they are able to guarantee certain level of confidence in data accuracy, wearable technology may certainly have to be used just as one of data points.
KS,
Great point on the accuracy of the Fitbits and manual data entry. I believe this is a weakness that new wearable technologies will face in the health care space.
Great post Tarran! I think it’s really interesting how pharma companies are taking advantage of the advancements in technology to help them make decisions earlier on.
I’d echo KS’ comment around data accuracy. I wonder if it would make sense for pharma companies to vertically integrate into some of these wearables in order to ensure quality assurance and accuracy. Is this a space you see them getting into? While it’s not necessarily their specialty, you see this happening across similar spaces. For example, Apple has acquired smaller health-tech related companies as they build out their Health app and Apple Watch with health sensors. Do you see pharma benefiting from doing something similar?
I think pharma’s involvement or investment in such technologies could persuade CROs to implement the use of this technology. QC and QA oversight from both pharma and CROs would definitely help improve confidence in the data. Great point!
As an early-adopter of wearables, I have always been bullish about “quantified health” and its potential to radically improve scientific research and advancement. I’m excited about the promise behind Litmus Health’s product, but I think a big initial challenge will be around data collection and accuracy (as mentioned in other comments). Based on Figure 2, it appears that Litmus Health will be relying on both “active” and “passive” data collection (i.e., user submitted inputs and pre-programmed, tracked data from Fitbit). While active data may offer more granular and specific insights, accuracy and reliability are often less robust due to users’ own biases (self-perception may not be objective) and behavior patterns (patients may not remember to log data correctly or consistently). Passive data is less susceptible to those pitfalls, but the pre-programmed nature restricts flexibility and limits customization, especially if it turns out that the tracked metrics are not relevant in evaluating a given drug’s efficacy. I’m curious to see how the company evolves to address these challenges.
Those are all great points and concerns that I also have. As the pool of data increases the hope is that data scientists are able to develop algorithms that account for the inherent error in active data collection.
I do not own a fitbit but I imagine the data from these wearables are typically used to help improve the health and lives of individuals. I always perceived wearables as trendy overly hyped products (not targeted to the medical field). This post, however identifies very important and potentially impactful uses of the data from these wearables (great job!). I am curious: since the data is being used for clinical trial and drug approval purposes, has anyone considered transitioning these consumer wearables to regulated medical devices? What does regulatory compliance look like for wearable technology?
Thanks for your comment. They have indeed transitioned wearables to medical devices. The first example that comes to mind are insulin pumps (https://myomnipod.com/), but there are many more that exist.
Thanks Tarran for the article! My friend and classmate won last year’s New Venture Competition’s Social Enterprise Track (http://www.hbs.edu/news/articles/Pages/ursure.aspx). His startup focuses on the need for adherence for preventative HIV medication. On a wider note, he enlightened me on the need for proper adherence to gather accurate patient information throughout the entire industry. As humans, we often forget to regularly take the medication, and sometimes our memories can get a bit fuzzy when reporting accurate adherence to our doctor. I see the technology you describe as a potentially powerful tool in the near future for addressing adherence gaps and the quality of the related data.
That’s a great point. Not only can this technology benefit the drug makers, but also benefit the patient. I would also take a look at eross’ blog post on AdhereTech that is using smart pill bottles to help with medication adherence.
This is an interesting example of how regulatory bodies and healthcare innovators can work together to help drive positive change. I was encouraged when I came across FDA approved applications for mobile phones (See http://www.healthitoutcomes.com/doc/fda-approves-apps-that-cut-readmissions-0001). The FDA’s flexibility in approaching healthcare IOT is commendable. I wonder if there is room for more cross-company collaboration in developing standards for how data can be collected, managed and analyzed as part of clinical trials that leverage healthcare IOT. Such standards could help accelerate the regulatory review process. The last thing we want is to slow-down the FDA with multiple competing platforms designed to speed up drug development in their own way!
One of the main issues with clinical trials is that there’s never really enough data to draw meaningful conclusion. When I was reading my first medical journals while in undergrad, I was genuinely appalled by the scarcity of data that was used to support far reaching conclusions. Sample sizes were either too smalls. Ridiculously broad assumptions were made. A big challenge was always whether or not data collected can / should be thought of as representative of a larger demographic. Furthermore, whether or not a drug succeeded was difficult to accurately assess because most of the participants in the trial were assumed to be the same (i.e. since they were randomly selected). This I always found misleading because it didn’t take into account the differences that the patients had when they were in the trial phase. Did the test patients end up exercising more? Did they eat differently? Did they sleep differently? etc. These are important questions that I’m glad can be addressed with new wearable technology
One of the main issues with clinical trials is that there’s never really enough data to draw meaningful conclusion. When I was reading my first medical journals while in undergrad, I was genuinely appalled by the scarcity of data that was used to support far reaching conclusions. Sample sizes were either too small. Ridiculously broad assumptions were made. After studying econometrics, I couldn’t help but gasp almost every time I read a medical journal!
A big challenge was always whether or not data collected can / should be thought of as representative of a larger demographic. Furthermore, whether or not a drug succeeded was difficult to accurately assess because most of the participants in the trial were assumed to be the same (i.e. since they were randomly selected). This I always found misleading because it didn’t take into account the differences that the patients had when they were in the trial phase. Did the test patients end up exercising more? Did they eat differently? Did they sleep differently? etc. These are important questions that I’m glad can be addressed with new wearable technology